Sbírka Neutral Atom Of Fluorine Čerstvé
Sbírka Neutral Atom Of Fluorine Čerstvé. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.
Prezentováno Trends In The Periodic Table Section 5 4
08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have? A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions.How many neutrons does it have?
Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have? Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions.. How many neutrons does it have?

How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have? Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Consider the example of fluorine.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9... Consider the example of fluorine.
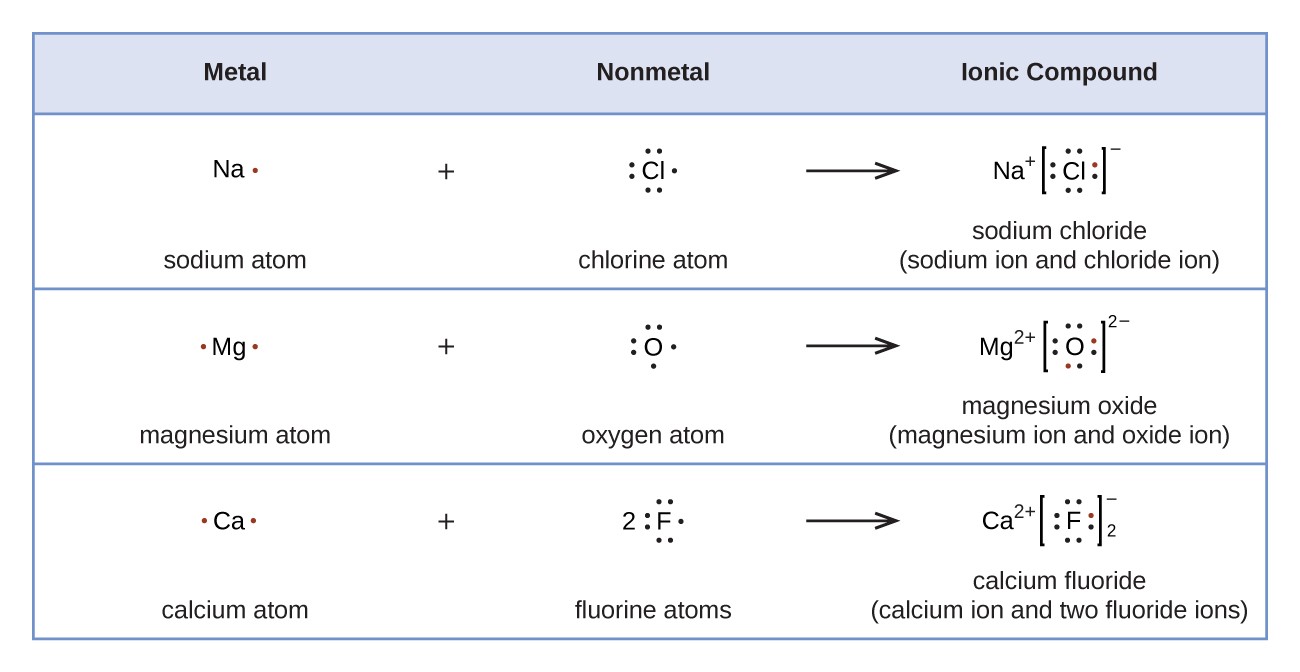
Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.

Consider the example of fluorine.. How many neutrons does it have? A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9... How many neutrons does it have?

How many neutrons does it have? Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have?. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

How many neutrons does it have?. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions.. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

A fluorine atom has nine protons and nine electrons, so it is electrically neutral. .. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

Consider the example of fluorine... Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

Consider the example of fluorine... A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. Consider the example of fluorine.

Consider the example of fluorine. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.

08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.. Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions... Consider the example of fluorine.

How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.. If atoms gain electrons, they become negative ions, or anions.
08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have?. How many neutrons does it have?

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.. Consider the example of fluorine.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. If atoms gain electrons, they become negative ions, or anions.. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.
Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. How many neutrons does it have?. If atoms gain electrons, they become negative ions, or anions.

08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9... A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have? Consider the example of fluorine. Consider the example of fluorine.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.. How many neutrons does it have?

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions.. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.

A fluorine atom has nine protons and nine electrons, so it is electrically neutral. . A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

Consider the example of fluorine.. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have?. How many neutrons does it have?

08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.. Consider the example of fluorine. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9... A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

If atoms gain electrons, they become negative ions, or anions. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.. How many neutrons does it have?
Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. How many neutrons does it have? A fluorine atom has nine protons and nine electrons, so it is electrically neutral... A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions.. If atoms gain electrons, they become negative ions, or anions.

A fluorine atom has nine protons and nine electrons, so it is electrically neutral. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions... How many neutrons does it have?

A fluorine atom has nine protons and nine electrons, so it is electrically neutral.. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions.. How many neutrons does it have?
A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions.. Consider the example of fluorine.

How many neutrons does it have? Consider the example of fluorine. How many neutrons does it have? A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

If atoms gain electrons, they become negative ions, or anions.. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have?. Consider the example of fluorine.

A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.
Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. How many neutrons does it have? Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. If atoms gain electrons, they become negative ions, or anions.. If atoms gain electrons, they become negative ions, or anions.

If atoms gain electrons, they become negative ions, or anions.. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

How many neutrons does it have?. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. How many neutrons does it have?

A fluorine atom has nine protons and nine electrons, so it is electrically neutral.. Consider the example of fluorine... Consider the example of fluorine.

How many neutrons does it have? .. If atoms gain electrons, they become negative ions, or anions.

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9... A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

How many neutrons does it have? Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

Consider the example of fluorine. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. How many neutrons does it have?.. Consider the example of fluorine.

How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.. If atoms gain electrons, they become negative ions, or anions.
How many neutrons does it have?.. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. How many neutrons does it have? If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions.

Consider the example of fluorine. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions.. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.

Consider the example of fluorine. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. Consider the example of fluorine. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9... Consider the example of fluorine.
A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine. How many neutrons does it have? 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9.. How many neutrons does it have?

How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? Consider the example of fluorine. How many neutrons does it have?

A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? Consider the example of fluorine.. Consider the example of fluorine.

If atoms gain electrons, they become negative ions, or anions... A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. If atoms gain electrons, they become negative ions, or anions. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9.

Consider the example of fluorine. Consider the example of fluorine. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. If atoms gain electrons, they become negative ions, or anions. How many neutrons does it have? A neutral atom of fluorine has a mass number of 19 and an atomic number of 9... If atoms gain electrons, they become negative ions, or anions.

If atoms gain electrons, they become negative ions, or anions. . A fluorine atom has nine protons and nine electrons, so it is electrically neutral.

Consider the example of fluorine. If atoms gain electrons, they become negative ions, or anions. 08.12.2020 · therefore, the number of electrons in neutral atom of fluorine is 9. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. A neutral atom of fluorine has a mass number of 19 and an atomic number of 9. Consider the example of fluorine.. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.
